Part a
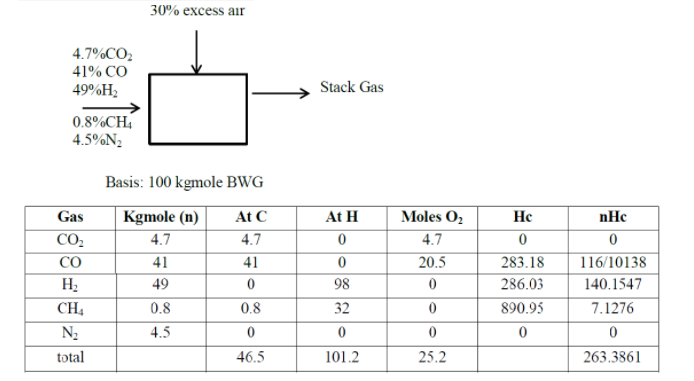
T=250C=298KPs=e73.649−2987258.2−7.3037ln(298)+4.1653∗10−6(2982))(101325760)=23.57torrN Dry BWD=100(760745−23.57∗0.9)(298273)=87.25kgmol BWGn dry gasnH2O=p dry gaspH2OnH2O=87.24(745−23.57∗0.923.57∗0.9)=2.556kgmoleO2 theo=46.5+4101.2−25.2=46.6kgmoleO2 supplied=1.30∗46.6=60.58kgmoleN2 air=60.58+2179=227.896kgmoleN2 total=N2 air+N2 fuel=227.896+4.5=232.4kgmoleCO2 formed=98∗46.6=41.33kgmoleCO formed=91∗46.6=5.167kgmoleUnburned H2=41∗5.167=1.2967kgmoleH2 combusted=101.2(21)−1.29167=49.3083kgmole=nH2O combustionNH2O from air=(60.58+227.896)(745−23.57∗0.8523.57∗0.85=7.9721kgmoleFree O2from=13.98+25.167+21.29167=17.2092kgmole
Part b
M3 air/m3 BWG=2.8848∗122.4∗745−23.57∗0.9760∗273298∗22.41∗298273∗760745−23.57∗0.9=2.88
Part c
100 moles BWGm3 stack gas=357.23∗122.4∗100101.325∗273573=17017.84kgmolm3
Part d
THTC∗1003025∗10083.33%


Comments